Topical Ibuprofen – Over the Counter (OTC) Product for the Treatment of Mild to Moderate Aches and Pains
Back to Clinical Programs Prevalence and Treatment Currently there are no topical ibuprofen products approved for use in the U.S. Topical Ibuprofen Completed phase 1 pharmacokinetic (PK) and safety trials with 7.5% Ibuprofen Cream clearly demonstrate proof-of-principle of the KNOSIS technology. The table below presents the PK data following a single application of 8.7 mL of 7.5% Ibuprofen Cream in normal healthy volunteers, demonstrating successful delivery of ibuprofen across the stratum corneum and into the systemic circulation. No significant systemic or dermal adverse effects were seen with any of the 7.5% Ibuprofen Cream treatment groups. Development Status and Partnering Opportunities
Topical Ibuprofen for the Treatment of Mild to Moderate Aches and Pains
NSAIDS are used by more than 30 million in the US alone. This is expected to increase as an aging population that is more active and working later in life combine to drive growth in the OTC marketplace. While proven effective in the treatment of pain and inflammation, oral ibuprofen is associated with a number of serious adverse effects, most notably increased risk for heart attack and stroke and gastrointestinal irritation (primarily due to inhibition of COX-1). Current oral preparations are mandated by the FDA to carry a black box warning explaining these risks.
SST's Ibuprofen Cream, 7.5%, is targeted to be an improved therapeutic approach for the over-the-counter (OTC) treatment of localized pain. It delivers therapeutic analgesic levels at the site of the inflamed tissue while keeping systemic plasma concentrations of ibuprofen extremely low, reducing and potentially eliminating the number of side effects commonly associated with the oral form.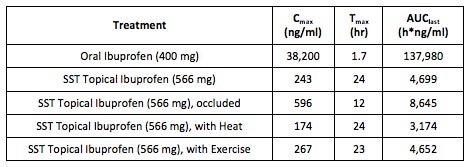
SST has completed Phase 2 trials and is actively engaged in partnering discussions. Please contact for more information regarding licensing and partnering opportunities.