Topical Sildenafil – Over the Counter (OTC) Product for the Treatment of Erectile Dysfunction (ED)
Back to Clinical Programs Prevalence and Treatment Sildenafil and other PDE5 inhibitors represent the most effective treatment of ED available today. In the US, these PDE5 inhibitors are available orally in varying doses, as prescription drugs. Although proven highly efficacious, oral PDE5 inhibitors have several adverse effects as well as significant drug-drug and drug-disease interactions in the target population. The most commonly reported adverse events include headache, flushing, dyspepsia, nasal congestion and impaired vision, including photophobia. In October 2007, the FDA announced that the labeling for all PDE5 inhibitors, including sildenafil, required a more prominent warning of the potential risk of sudden hearing loss. In addition, major drug-drug interactions occur with other PDE and PDE5 inhibitors such as Riociquat and nitrates as well as protease inhibitors, and potent CYP450 3A4 inhibitors. Of special concern is the interaction with nitrates causing unsafe hypotension. Moderate drug-drug interactions include warfarin and alpha blockers. These as well as major/moderate disease interactions (cardiovascular, renal dysfunction and pulmonary, alcoholism, hearing loss, liver disease, priapism, retinitis pigmentosa and seizure disorders) significantly impact the number of patients that can benefit from oral sildenafil use. It is estimated that approximately 18% percent of patients are contraindicated due to either medications or disease. Topical Sildenafil Cream A completed phase 1 pharmacokinetic (PK) and safety trial with Sildenafil Cream, 3.6% clearly demonstrates proof-of-principle of the KNOSIS technology. The table below presents the PK data following a single application of 2 grams of Sildenafil Cream, 3.6% (occluded and non-occluded) as compared to a single oral dose of 50 mg Viagra® in normal healthy male volunteers, demonstrating successful delivery of sildenafil across the skin and into the systemic circulation. No significant systemic or dermal adverse effects were seen with either of the Sildenafil Cream, 3.6% treatment groups. Given the very low systemic levels (relative to oral sildenafil), we expect Sildenafil Cream, 3.6% to be very well tolerated without the systemic side effects typically experienced with oral PDE5 inhibitors. Development Status and Partnering Opportunities
Topical Sildenafil for the Treatment of Erectile Dysfunction (ED)
Erectile Dysfunction (ED), the persistent inability to achieve or maintain an erection sufficient for satisfactory sexual performance, is estimated to affect up to 20 million men in the US; with approximately 5 million diagnosed and 600,000 new cases diagnosed each year. The global market is estimated at over $4B. The disorder is age-associated with estimated prevalence rates of 39% among men 40 years old and up to 70% percent among those 70 years old.
SST's topical formulation of Sildenafil Cream, 3.6% offers the potential for improved safety, convenience and accessibility, and is targeted to be first ever FDA approved over-the-counter (OTC) product for the safe and effective treatment of ED. Additionally, Sildenafil Cream, 3.6% offers the potential for several clinical advantages over oral PDE5 inhibitors including more targeted local delivery at required therapeutic levels and faster onset of action.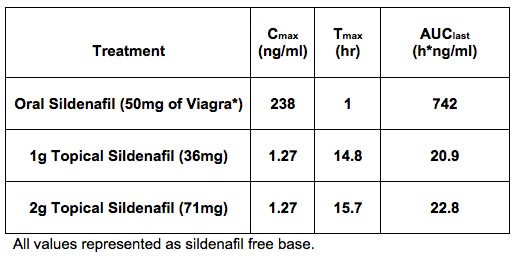
SST has recently completed a Phase 2 proof-of-concept study in men with mild to moderate ED and is actively engaged in partnering discussions. Please contact
for more information regarding licensing and partnering opportunities.